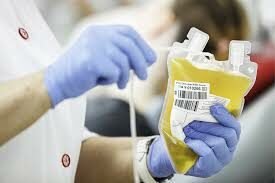
Evidences have been found that seriously ill coronavirus patients can benefit from infusions of blood plasma collected from people who have recovered from COVID19.
Two teams of medics working at separate hospitals in China gave antibody-rich plasma to 15 severely ill patients and recorded striking improvements in many of them.
In one pilot study, doctors in Wuhan gave “convalescent plasma” to 10 severely ill patients and found that virus levels in their bodies dropped rapidly. Within three days, the doctors saw improvements in the patients’ symptoms, ranging from shortness of breath and chest pains to fever and coughs.
The US Food and Drug Administration (FDA) is also looking for plasma treatment.
The FDA on Friday announced a programme with “expanded access” which will provide convalescent plasma to patients nationwide, collected and distributed by the American Red Cross.
One donation can generate nearly four units of plasma and could possibly help up to four people; however, it is not yet known to be a safe and effective treatment for COVID-19.
The clinical trials are led by the Minnesota-based Mayo Clinic, who will examine whether antibody therapy may shorten the duration of illness, the severity of the disease, or prevent people from dying.
The therapy may prove to be a useful method in boosting the immune system of front-line health workers.
The effort is also underway at Stamford Health — which is conducting Connecticut’s first clinical trial — and at Nuvance Health in Danbury and Norwalk, which is collecting the plasma of recovered COVID-19 patients for a similar study later this month.
“Plasma therapy has a long history of success in helping patients with diseases such as polio, measles and even the flu in their recovery” said Dr. Paul Sachs, director of pulmonary medicine at Stamford Health and a principal investigator for the trial. “Pilot studies at other organizations have shown promise, and we hope that we’ll see those successes replicated for COVID-19.”
Stamford Health, which runs Stamford Hospital, has about 30 people sick with coronavirus who are eligible for the plasma therapy, several of whom could receive the simple treatment as soon as the end of the week.
“The antibodies are inside the plasma and the plasma is transfused directly into sick patients,” said Suzanne Rose, Stamford Health’s Office of Research director, and the study sub-investigator. “This could be revolutionary, because there’s very few side effects, and we don’t have to give somebody drugs to help their body fight harder against the infection.”
The treatment, known as “convalescent plasma” involves taking the liquid component of blood from those who have recovered and transfusing it into critically ill coronavirus patients. It was used during the Spanish flu epidemic of 1918.Although not a cure, the therapy has the potential to lessen the severity of a patient’s symptoms.
The therapy relies on the fact that people who have recovered from a viral infection have antibodies in their blood that can rapidly detect and destroy the virus the next time it attacks. Infusing the plasma into patients, and potentially into people at risk of being infected, can boost their immune systems and potentially provide protection.