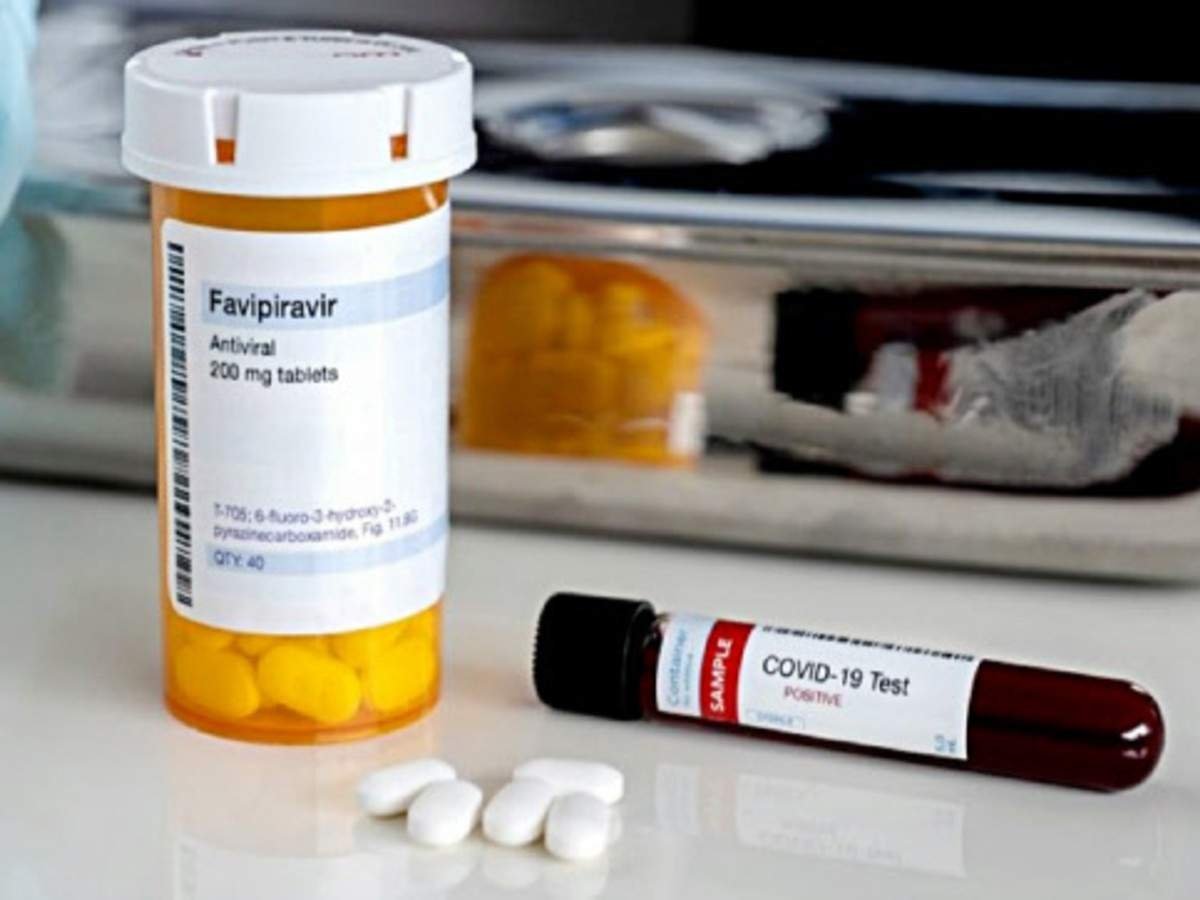
Glenmark Pharmaceuticals has launched antiviral drug Favipiravir, under the brand name FabiFlu, for the treatment of patients with mild to moderate COVID-19.
The company has received manufacturing and marketing approval from the Drugs Controller General of India (DCGI) yesterday.
FabiFlu is the first oral Favipiravir-approved medication for the treatment of COVID-19, the company said in a statement.
“This approval comes at a time when cases in India are spiralling like never before, putting a tremendous pressure on our healthcare system,” Glenmark Pharmaceuticals Chairman and Managing Director Glenn Saldanha said.
Each tablet of 200 mg costs ₹103 and a strip containing 34 tablets has been priced at ₹3500. It will be made available at hospitals and chemist shops and will be sold under medical presciption.
On the first day, a patient will be administered 1800 mg twice each. From the second day, the patient will be given 800 mg twice a day for upto 14 days as per the doctor’s advice. The medicine can be administered to patients between the age group of 18 and 75.
Since the approval is under emergency category due to the pandemic, the patient has to give an undertaking before consuming the medicine.
The company hopes that the availability of an effective treatment such as FabiFlu will considerably help assuage this pressure, and offer patients in India a much needed and timely therapy option, he added.
Glenmark is the first company to come out with an oral antiviral drug for mild and moderate Covid-19 treatment.
Favipiravir has been used in Russia, Japan and China to treat such patients and has been proved successful.